12. Molecular Shapes & Valence Bond Theory
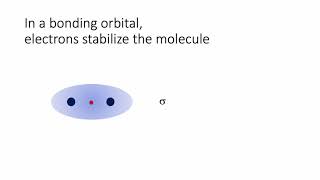
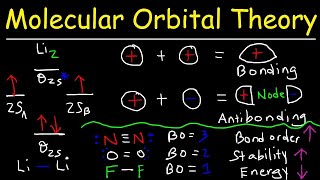
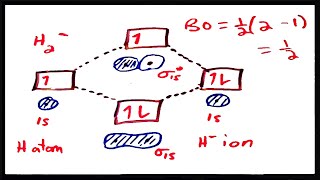
MO Theory: Bond Order
Problem 98
Textbook Question
Textbook QuestionUse the MO energy diagram in Figure 8.22b to describe the bonding in O2+, O2, and O2-. Which of the three is likely to be stable? What is the bond order of each? Which contain unpaired electrons?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
12mPlay a video:
866
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos