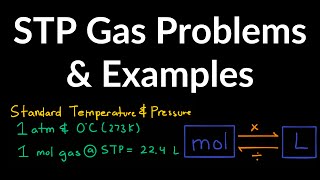
7. Gases
Gas Stoichiometry
Problem 141
Textbook Question
Textbook QuestionWhen 10.0 g of a mixture of Ca1ClO322 and Ca1ClO22 is heated to 700 °C in a 10.0-L vessel, both compounds decompose, forming O21g2 and CaCl21s2. The final pressure inside the vessel is 1.00 atm. (b) What is the mass of each compound in the original mixture?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
19mPlay a video:
525
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos