8. Thermochemistry
Heat Capacity
Problem 101
Textbook Question
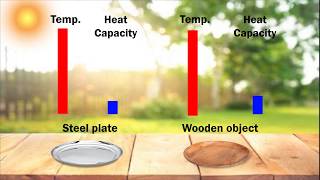
Textbook QuestionUse standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The ΔH °f for H2O(s) is -291.8 kJ/mol.) Use this value to calculate the mass of ice required to cool 355 mL of a beverage from room temperature (25.0 °C) to 0.0 °C. Assume that the specific heat capacity and density of the beverage are the same as those of water.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3961
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos