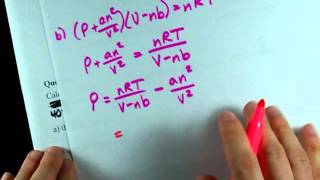7. Gases
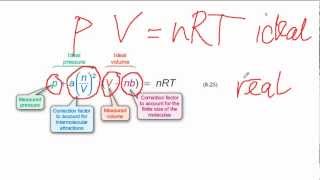
Van der Waals Equation
Problem 94a
Textbook Question
Textbook QuestionCalculate the pressure that CCl4 will exert at 80 °C if 1.00 mol occupies 33.3 L, assuming that (a) CCl4 obeys the ideal-gas equation (b) CCl4 obeys the van der Waals equation. (Values for the van der Waals constants are given in Table 10.3.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
584
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos