8. Thermochemistry
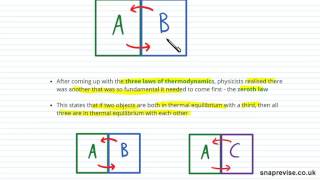
Thermal Equilibrium
Problem 69
Textbook Question
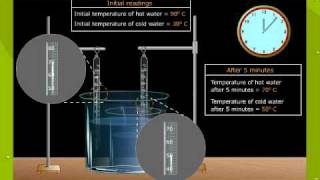
Textbook QuestionTwo substances, A and B, initially at different temperatures, come into contact and reach thermal equilibrium. The mass of substance A is 6.15 g and its initial temperature is 20.5 °C. The mass of substance B is 25.2 g and its initial temperature is 52.7 °C. The final temperature of both substances at thermal equilibrium is 46.7 °C. If the specific heat capacity of substance B is 1.17 J>g # °C, what is the specific heat capacity of substance A?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3042
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos