2. Atoms & Elements
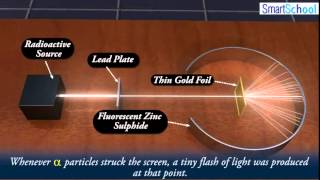
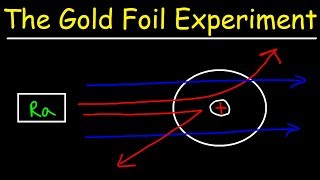
Rutherford Gold Foil Experiment

Get help from an AI Tutor
Ask a question to get started.
Problem 73
Textbook Question
Textbook QuestionThe atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1065
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos