Textbook Question
The C2 molecule can be represented by an MO diagram similar to that in Figure 8.22a. (b) To increase the bond order of C2, should you add or remove an electron?
1531
views

 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 106a
Problem 106a Verified step by step guidance
Verified step by step guidance


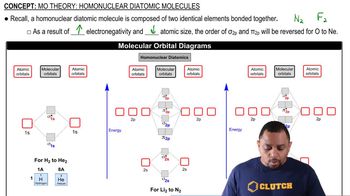
At high temperatures, sulfur vapor is predominantly in the form of S2(g) molecules. (d) When two electrons are added to S2, the disulfide ion S22- is formed. Is the bond length in S22- likely to be shorter or longer than the bond length in S2? Explain.
Carbon monoxide is produced by incomplete combustion of fossil fuels. (a) Give the electron configuration for the valence molecular orbitals of CO. The orbitals have the same energy order as those of the N2 molecule.
Carbon monoxide is produced by incomplete combustion of fossil fuels. (b) Do you expect CO to be paramagnetic or diamagnetic?