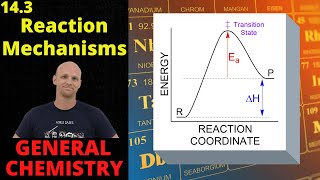
15. Chemical Kinetics
Reaction Mechanism
Problem 109b
Textbook Question
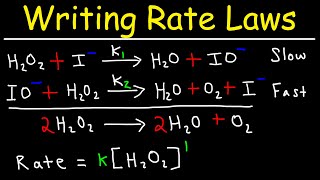
Textbook QuestionThe gas-phase decomposition of ozone is thought to occur by the following two-step mechanism. Step 1: O31g2ΔO21g2 + O1g2 (fast) (b) Derive the rate law that is consistent with this mechanism. (Hint: The product appears in the rate law.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
760
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos