16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 158
Textbook Question
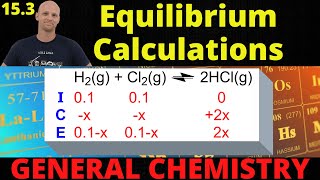
Textbook QuestionA 14.58 g quantity of N2O4 was placed in a 1.000-L reaction vessel at 400 K. The N2O4 decomposed to an equilibrium mix- ture of N2O4 and NO2 that had a total pressure of 9.15 atm. (b) How much heat (in kilojoules) was absorbed when the N2O4 decomposed to give the equilibrium mixture? (Stan- dard heats of formation may be found in Appendix B.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
279
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos