3. Chemical Reactions
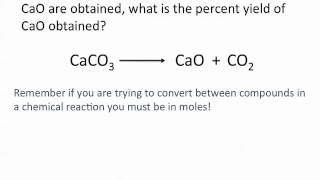
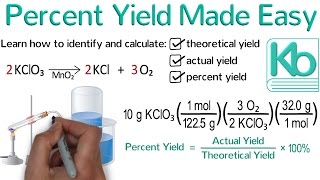
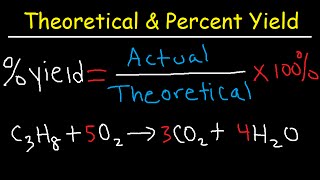
Percent Yield
Problem 84
Textbook Question
Textbook QuestionWhen ethane 1C2H62 reacts with chlorine 1Cl22, the main product is C2H5Cl, but other products containing Cl, such as C2H4Cl2, are also obtained in small quantities. The formation of these other products reduces the yield of C2H5Cl. (a) Calculate the theoretical yield of C2H5Cl when 125 g of C2H6 reacts with 255 g of Cl2, assuming that C2H6 and Cl2 react only to form C2H2Cl and HCl. (b) Calculate the percent yield of C2H5Cl if the reaction produces 206 g of C2H5Cl.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1064
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos