15. Chemical Kinetics
Reaction Mechanism
Problem 110a
Textbook Question
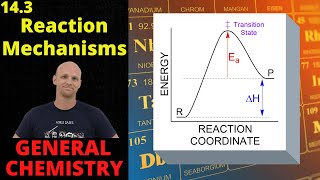
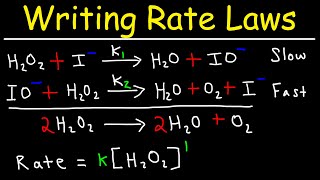
Textbook QuestionThe thermal decomposition of nitryl chloride, NO2Cl, is believed to occur by the following mechanism: NO2Cl1g2 ¡ k1 NO21g2 + Cl1g2 Cl1g2 + NO2Cl1g2 ¡ k2 NO21g2 + Cl21g2 (c) What rate law is predicted by this mechanism if the first step is rate-determining?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
729
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos