6. Chemical Quantities & Aqueous Reactions
Solution Stoichiometry
Problem 129
Textbook Question
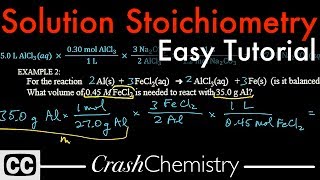
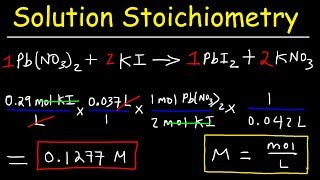
Textbook QuestionStandardized solutions of KBrO3 are frequently used in redox titrations. The necessary solution can be made by dissolving KBrO3 in water and then titrating it with an As(III) solution. What is the molar concentration of a KBrO3 solution if 28.55 mL of the solution is needed to titrate 1.550 g of As2O3? See Problem 4.128 for the balanced equation. (As2O3 dissolves in aqueous acid solution to yield H3AsO3: As2O3 + 3 H2OS 2 H3AsO3.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
869
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos