7. Gases
Gas Stoichiometry
Problem 72a
Textbook Question
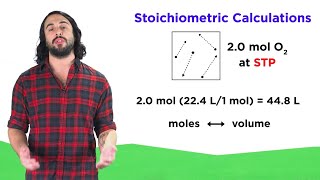
Textbook QuestionHydrogen gas can be prepared by reaction of zinc metal with aqueous HCl: Zn1s2 + 2 HCl1aq2¡ZnCl21aq2 + H21g2 (a) How many liters of H2 would be formed at 742 mm Hg and 15 °C if 25.5 g of zinc was allowed to react?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
623
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos