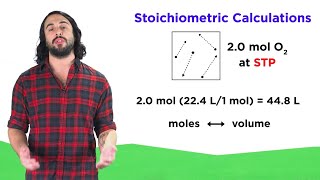
7. Gases
Gas Stoichiometry
Problem 121
Textbook Question
Textbook QuestionA 4.00-g sample of a mixture of CaO and BaO is placed in a 1.00-L vessel containing CO2 gas at a pressure of 97.33 kPa and a temperature of 25 °C. The CO2 reacts with the CaO and BaO, forming CaCO3 and BaCO3. When the reaction is complete, the pressure of the remaining CO2 is 20.0 kPa. (b) Calculate the mass percentage of CaO in the mixture.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
13mPlay a video:
1129
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos