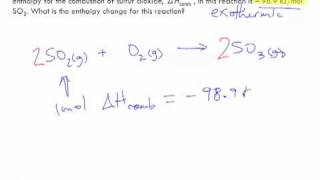8. Thermochemistry
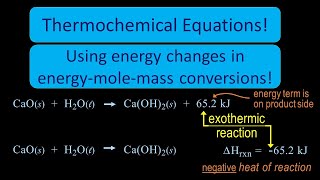
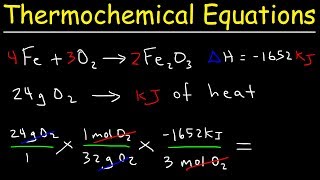
Thermochemical Equations
Problem 46b
Textbook Question
Textbook QuestionAt one time, a common means of forming small quantities of oxygen gas in the laboratory was to heat KClO3: 2 KClO31s2¡2 KCl1s2 + 3 O21g2 H = -89.4 kJ For this reaction, calculate H for the formation of (a) 1.36 mol of O2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
515
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos