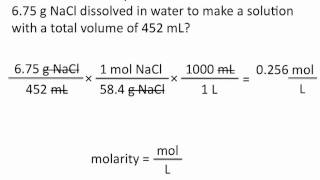
6. Chemical Quantities & Aqueous Reactions
Molarity
Problem 67
Textbook Question
Textbook Question(a) How many grams of ethanol, CH3CH2OH, should you dissolve in water to make 1.00 L of vodka (which is an aqueous solution that is 6.86 M ethanol)? (b) Using the density of ethanol (0.789 g/mL), calculate the volume of ethanol you need to make 1.00 L of vodka.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1524
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos