9. Quantum Mechanics
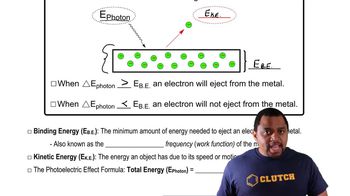
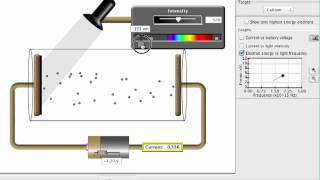
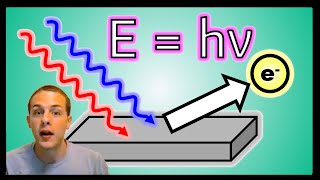
Photoelectric Effect
Problem 52
Textbook Question
Textbook QuestionThe work function of cesium metal is 188 kJ/mol, which corresponds to light with a wavelength of 637 nm. Which of the following will cause the smallest number of electrons to be ejected from cesium? (a) High-amplitude wave with a wavelength of 500 nm (b) Low-amplitude wave with a wavelength of 500 nm (c) High-amplitude wave with a wavelength of 650 nm (d) Low-amplitude wave with a wavelength of 650 nm
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
1073
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos