6. Chemical Quantities & Aqueous Reactions
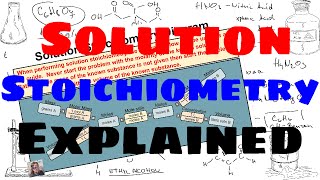
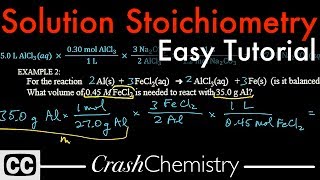
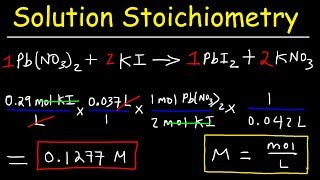
Solution Stoichiometry
Problem 142
Textbook Question
Textbook QuestionMorphine has the formula C17H19NO3. It is a base and accepts one proton per molecule. It is isolated from opium. A 0.682-g sample of opium is found to require 8.92 mL of a 0.0116 M solution of sulfuric acid for neutralization. Assuming that morphine is the only acid or base present in opium, calculate the percent morphine in the sample of opium.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
4270
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos