16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 51
Textbook Question
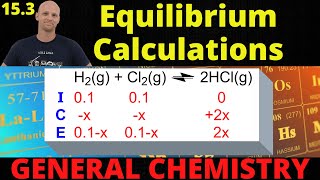
Textbook QuestionConsider the reaction and the associated equilibrium constant: aA(g) ⇌ bB(g) Kc = 4.0 Find the equilibrium concentrations of A and B for each value of a and b. Assume that the initial concentration of A in each case is 1.0 M and that no B is present at the beginning of the reaction. c. a=1;b=2
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
1893
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos