16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 77
Textbook Question
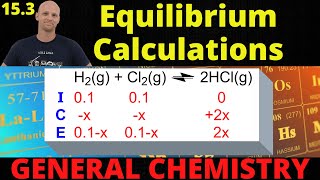
Textbook QuestionAt 650 K, the reaction MgCO3(s) ⇌ MgO(s) + CO2(g) has Kp = 0.026. A 10.0-L container at 650 K has 1.0 g of MgO(s) and CO2 at P = 0.0260 atm. The container is then compressed to a volume of 0.100 L. Find the mass of MgCO3 that is formed.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1859
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos