6. Chemical Quantities & Aqueous Reactions
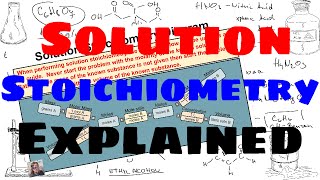
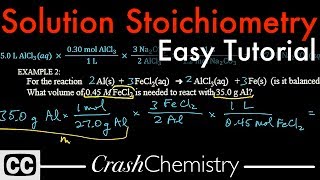
Solution Stoichiometry
Problem 69
Textbook Question
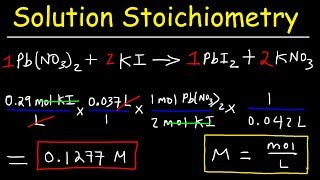
Textbook QuestionA 25.0-mL sample of a 1.20 M potassium chloride solution is mixed with 15.0 mL of a 0.900 M lead(II) nitrate solution and this precipitation reaction occurs: 2 KCl(aq) + Pb(NO3)2(aq)¡ PbCl2(s) + 2 KNO3(aq) The solid PbCl2 is collected, dried, and found to have a mass of 2.45 g. Determine the the percent yield.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
3405
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos