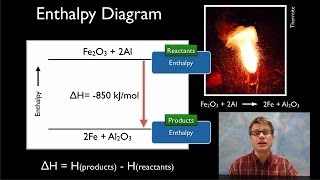
8. Thermochemistry
Enthalpy of Formation

Get help from an AI Tutor
Ask a question to get started.
Problem 86
Textbook Question
Textbook QuestionPentane (C5H12) is a component of gasoline that burns according to the following balanced equation: C5H12(l ) + 8 O2( g)¡5 CO2( g) + 6 H2O( g) Calculate ΔH °rxn for this reaction using standard enthalpies of formation. (The standard enthalpy of formation of liquid pentane is -146.8 kJ>mol.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
7228
views
3
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 6 videos