9. Quantum Mechanics
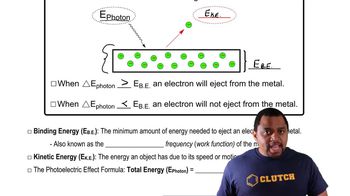
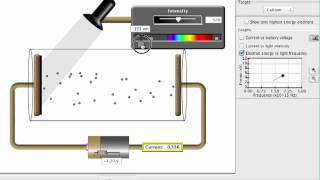
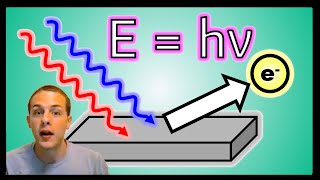
Photoelectric Effect
Problem 55
Textbook Question
Textbook QuestionCesium metal is frequently used in photoelectric cells because the amount of energy necessary to eject electrons from a cesium surface is relatively small—only 206.5 kJ/mol. What wavelength of light in nanometers does this correspond to?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1844
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos