7. Gases
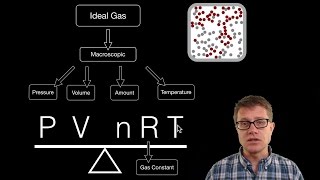
The Ideal Gas Law
Problem 76
Textbook Question
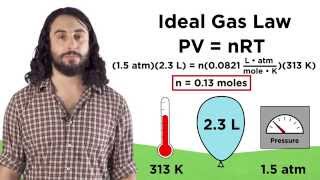
Textbook QuestionA typical high-pressure tire on a bicycle might have a volume of 365 mL and a pressure of 7.80 atm at 25 °C. Suppose the rider filled the tire with helium to minimize weight. What is the mass of the helium in the tire?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1243
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos