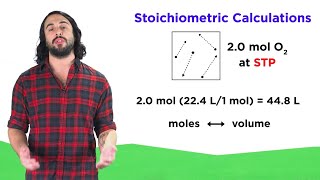
7. Gases
Gas Stoichiometry
Problem 78
Textbook Question
Textbook QuestionOzone is depleted in the stratosphere by chlorine from CF3Cl according to this set of equations: CF3Cl + UV light¡CF3 + Cl Cl + O3¡ClO + O2 O3 + UV light¡O2 + O ClO + O¡Cl + O2 What total volume of ozone at a pressure of 25.0 mmHg and a temperature of 225 K is destroyed when all of the chlorine from 15.0 g of CF3 Cl goes through 10 cycles of the given reactions?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1388
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos