12. Molecular Shapes & Valence Bond Theory
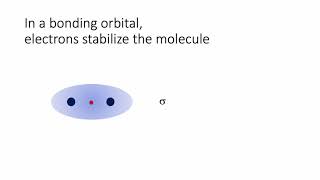
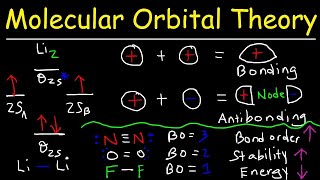
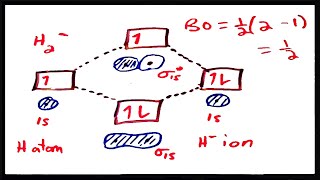
MO Theory: Bond Order
Problem 76d
Textbook Question
Textbook QuestionUsing the molecular orbital energy ordering for second-row homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? a. 10
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
383
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos