7. Gases
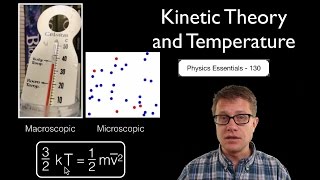
Kinetic Molecular Theory
Problem 84
Textbook Question
Textbook QuestionAt constant pressure, the mean free path 1l2 of a gas molecule is directly proportional to temperature. At constant temperature, l is inversely proportional to pressure. If you compare two different gas molecules at the same temperature and pressure, l is inversely proportional to the square of the diameter of the gas molecules. Put these facts together to create a formula for the mean free path of a gas molecule with a proportionality constant (call it Rmfp, like the ideal-gas constant) and define units for Rmfp.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
834
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos