16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 53
Textbook Question
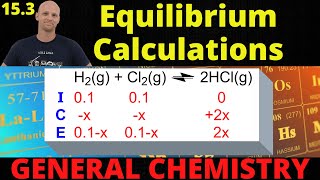
Textbook QuestionFor the reaction shown here, Kc = 0.513 at 500 K. N2O4(g) ⇌ 2NO2(g) If a reaction vessel initially contains an N2O4 concentration of 0.0500 M at 500 K, what are the equilibrium concentrations of N2O4 and NO2 at 500 K?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1676
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos