1. Intro to General Chemistry
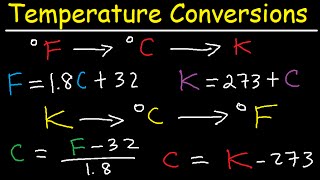
Temperature
Problem 58a
Textbook Question
Textbook QuestionSuppose you were dissatisfied with both Celsius and Fahrenheit units and wanted to design your own temperature scale based on ethyl alcohol (ethanol). On the Celsius scale, ethanol has a melting point of -117.3 °C and a boiling point of 78.5 °C, but on your new scale calibrated in units of degrees ethanol, °E, you define ethanol to melt at 0 °E and boil at 200 °E. (c) What are the melting and boiling points of water on the ethanol scale?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1427
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos