15. Chemical Kinetics
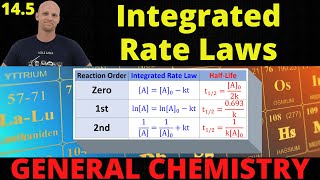
Integrated Rate Law
Problem 91
Textbook Question
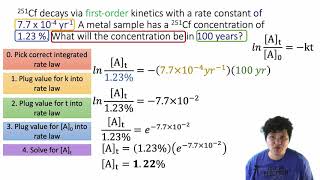
Textbook QuestionIodine atoms combine to form I2 in liquid hexane solvent with a rate constant of 1.5 * 1010 L>mol # s. The reaction is second order in I. Since the reaction occurs so quickly, the only way to study the reaction is to create iodine atoms almost instantaneously, usually by photochemical decomposition of I2. Suppose a flash of light creates an initial [I] concentration of 0.0100 M. How long will it take for 95% of the newly created iodine atoms to recombine to form I2?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
2040
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos