6. Chemical Quantities & Aqueous Reactions
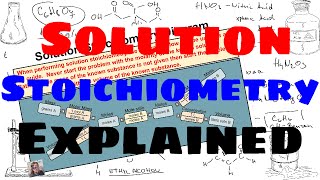
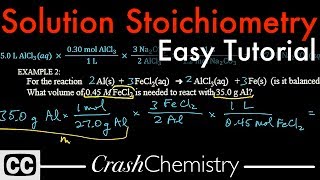
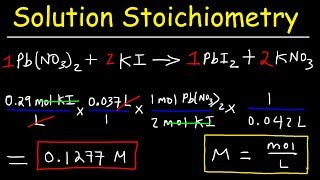
Solution Stoichiometry
Problem 102b
Textbook Question
Textbook QuestionCitric acid, C6H8O7, is a triprotic acid. It occurs naturally in citrus fruits like lemons and has applications in food flavouring and preservatives. A solution containing an unknown concentration of the acid is titrated with KOH. It requires 23.20 mL of 0.500 M KOH solution to titrate all three acidic protons in 100.00 mL of the citric acid solution. Calculate the molarity of the citric acid solution.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1940
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos