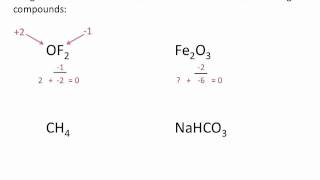6. Chemical Quantities & Aqueous Reactions
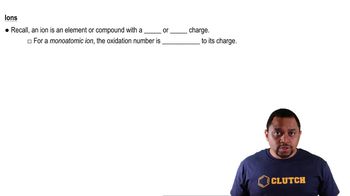
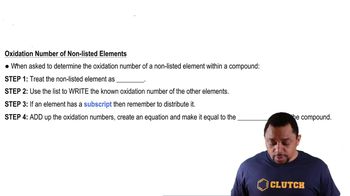
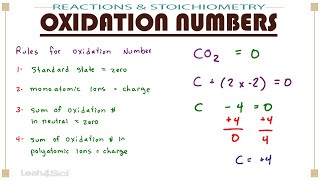
Calculate Oxidation Numbers

Get help from an AI Tutor
Ask a question to get started.
Problem 106
Textbook Question
Textbook QuestionDisulfides are compounds that have S ¬ S bonds, like peroxides have O ¬ O bonds. Thiols are organic compounds that have the general formula R ¬ SH, where R is a generic hydrocarbon. The SH- ion is the sulfur counterpart of hydroxide, OH-. Two thiols can react to make a disulfide, R ¬ S ¬ S ¬ R. (b) What is the oxidation state of sulfur in a disulfide?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
464
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 16 videos