7. Gases
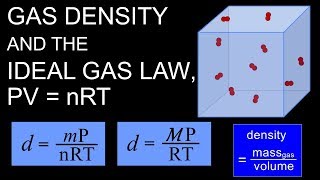
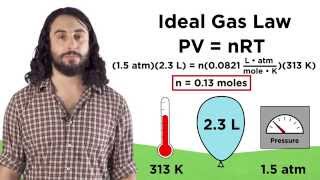
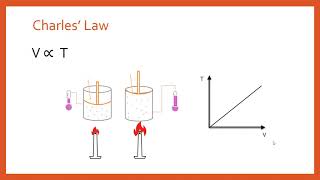
The Ideal Gas Law Derivations
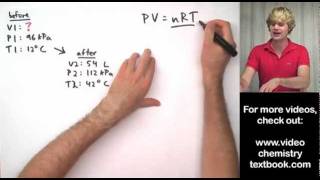
Problem 48a
Textbook Question
Textbook QuestionAssume that you have a cylinder with a movable piston. What would happen to the gas pressure inside the cylinder if you were to do the following? (a) Triple the Kelvin temperature while holding the volume constant
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
481
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos