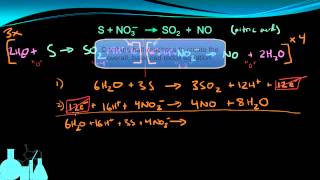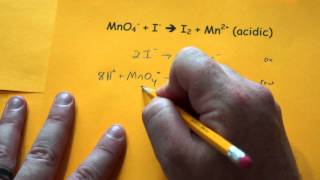6. Chemical Quantities & Aqueous Reactions
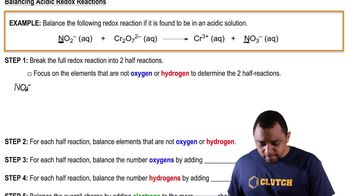
Balancing Redox Reactions: Acidic Solutions
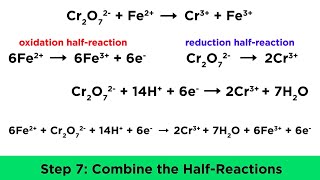
Problem 63
Textbook Question
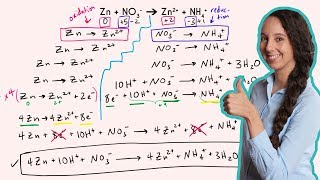
Textbook QuestionA galvanic cell is constructed from a Zn/Zn2+ half-cell (anode) and a Cl2/Cl- half-cell (cathode). (b) Write balanced equations for the electrode and overall cell reactions.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
797
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos