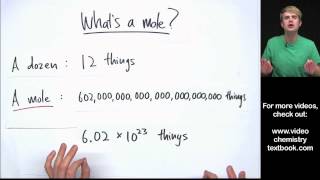2. Atoms & Elements
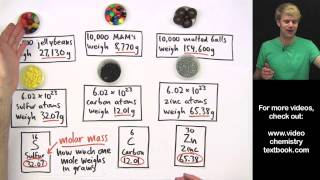
Mole Concept
Problem 36d
Textbook Question
Textbook QuestionCalculate the following quantities: (d) number of O atoms in 6.25⨉10−3 mol Al(NO3)3
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
768
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos