16. Chemical Equilibrium
ICE Charts

Get help from an AI Tutor
Ask a question to get started.
Problem 92
Textbook Question
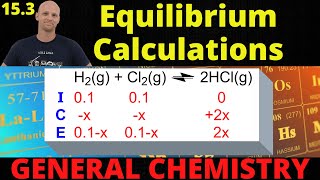
Textbook QuestionConsider the hypothetical reaction A1g2 + 2 B1g2 Δ 2 C1g2, for which Kc = 0.25 at a certain temperature. A 1.00-L reaction vessel is loaded with 1.00 mol of compound C, which is allowed to reach equilibrium. Let the variable x represent the number of mol>L of compound A present at equilibrium. (e) From the plot in part (d), estimate the equilibrium concentrations of A, B, and C. (Hint: You can check the accuracy of your answer by substituting these concentrations into the equilibrium expression.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
10mPlay a video:
335
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos