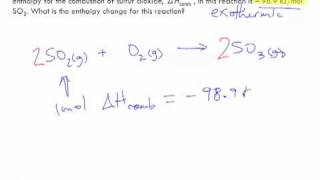8. Thermochemistry
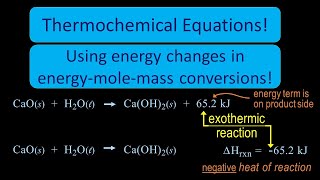
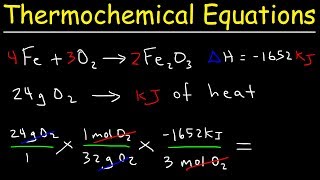
Thermochemical Equations
Problem 61
Textbook Question
Textbook QuestionNitromethane (CH3NO2) burns in air to produce significant amounts of heat. 2 CH3NO2(l ) + 32 O2( g)¡2 CO2( g) + 3 H2O(l ) + N2( g) ΔH °rxn = -1418 kJ How much heat is produced by the complete reaction of 5.56 kg of nitromethane?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
2694
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos