2. Atoms & Elements
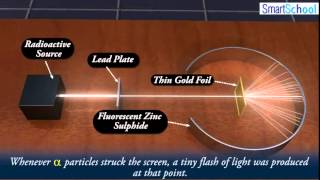
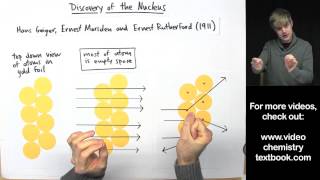
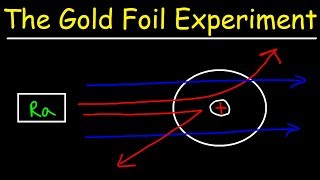
Rutherford Gold Foil Experiment
Problem 42
Textbook Question
Textbook QuestionWhich statements are inconsistent with Rutherford's nuclear theory as it was originally stated? Why? a. Since electrons are smaller than protons, and since a hydrogen atom contains only one proton and one electron, it must follow that the volume of a hydrogen atom is mostly due to the proton. b. A nitrogen atom has 7 protons in its nucleus and 7 electrons outside of its nucleus. c. A phosphorus atom has 15 protons in its nucleus and 150 electrons outside of its nucleus. d. The majority of the mass of a fluorine atom is due to its 9 electrons.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1387
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos