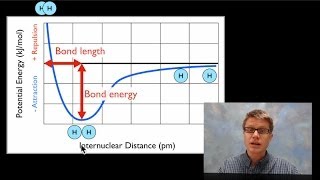
11. Bonding & Molecular Structure
Bond Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 123
Textbook Question
Textbook QuestionUse average bond enthalpies from Table 8.4 to estimate the enthalpies of the following gas-phase reactions: Reaction 1: HF1g2 + H2O1g2 Δ F-1g2 + H3O+1g2 Reaction 2: HCl1g2 + H2O1g2 Δ Cl-1g2 + H3O+1g2 Are both reactions exothermic? How do these values relate to the different strengths of hydrofluoric and hydrochloric acid?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
9mPlay a video:
1133
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos