3. Chemical Reactions
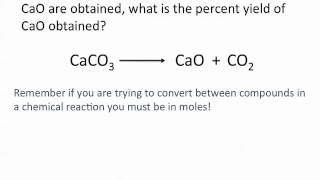
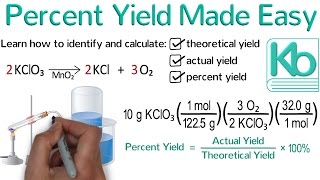
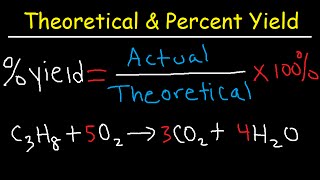
Percent Yield
Problem 51
Textbook Question
Textbook QuestionUrea (CH4N2O) is a common fertilizer that is synthesized by the reaction of ammonia (NH3) with carbon dioxide: 2 NH3(aq) + CO2(aq)¡CH4N2O(aq) + H2O(l ) In an industrial synthesis of urea, a chemist combines 136.4 kg of ammonia with 211.4 kg of carbon dioxide and obtains 168.4 kg of urea. Determine the limiting reactant. Determine the theoretical yield of urea. Determine the percent yield for the reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
7347
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos