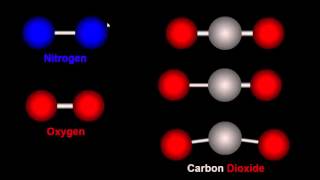
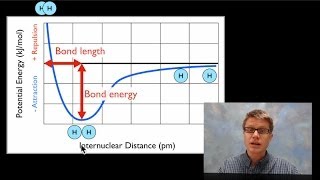
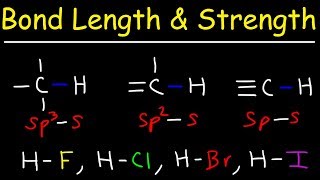11. Bonding & Molecular Structure
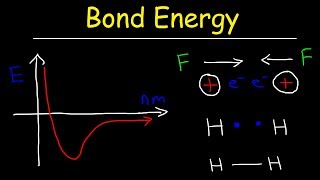
Bond Energy
Problem 42
Textbook Question
Textbook QuestionExplain the difference in the bond dissociation energies for the following bonds: (C-F, 450 kJ/mol), (N-F, 270 kJ/mol), (O-F, 180 kJ/mol), (F-F, 159 kJ/mol).
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1047
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos