19. Chemical Thermodynamics
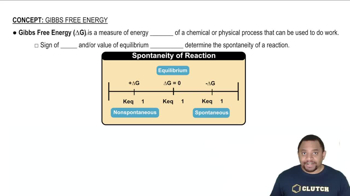
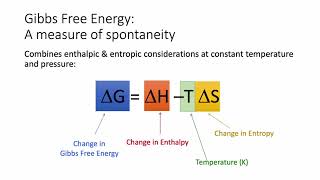
Gibbs Free Energy
Problem 46
Textbook Question
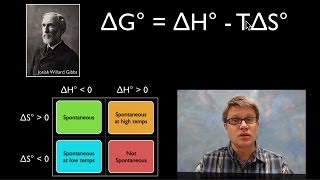
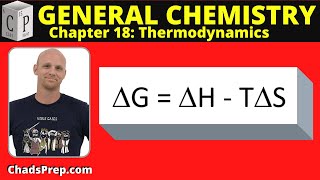
Textbook QuestionCalculate the free energy change for this reaction at 25 °C. Is the reaction spontaneous? (Assume that all reactants and products are in their standard states.) 2 Ca(s) + O2( g) ¡ 2 CaO(s) ΔH° rxn = -1269.8 kJ; ΔSrxn ° = -364.6 J>K
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
4689
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos