7. Gases
The Ideal Gas Law
Problem 107a
Textbook Question
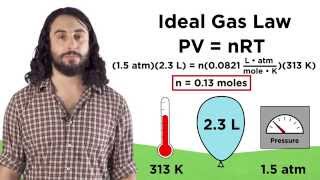
Textbook QuestionOlympic cyclists fill their tires with helium to make them lighter. Calculate the mass of air in an air-filled tire and the mass of helium in a helium-filled tire. Assume that the volume of the tire is 855 mL, that it is filled to a total pressure of 125 psi, and that the temperature is 25 °C. Also, assume an average molar mass for air of 28.8 g>mol. Calculate the mass of helium in a helium-filled tire.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
1779
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos