8. Thermochemistry
Heat Capacity
Problem 50b
Textbook Question
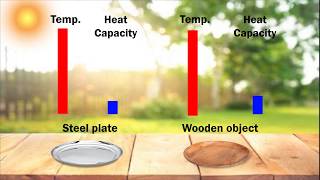
Textbook QuestionTwo solid objects, A and B, are placed in boiling water and allowed to come to the temperature of the water. Each is then lifted out and placed in separate beakers containing 1000 g of water at 10.0 °C. Object A increases the water temperature by 3.50 °C; B increases the water temperature by 2.60 °C. (a) Which object has the larger heat capacity?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1466
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 14 videos