10. Periodic Properties of the Elements
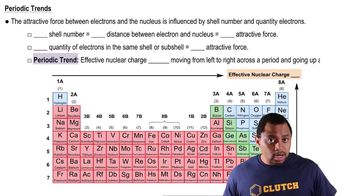
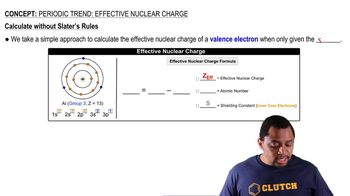
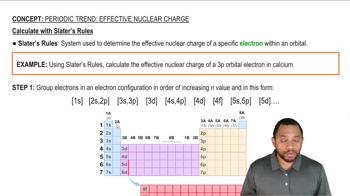
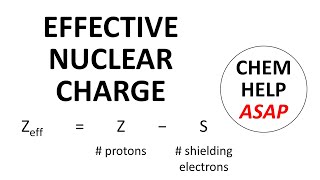
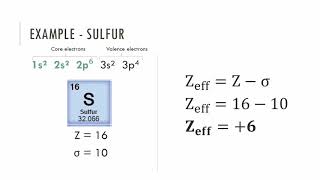
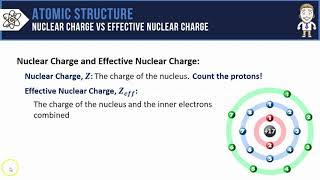
Periodic Trend: Effective Nuclear Charge

Get help from an AI Tutor
Ask a question to get started.
Problem 12
Textbook Question
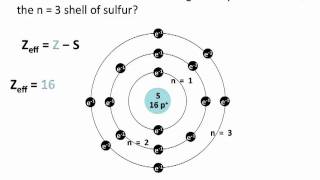
Textbook QuestionFor a multielectron atom, a 3s orbital lies lower in energy than a 3p orbital because (LO 5.16) (a) a 3p orbital has more nodal surfaces than a 3s orbital. (b) an electron in a 3p orbital has a higher probability of being closer to the nucleus than an electron in a 3s orbital. (c) inner electrons shield electrons in a 3p orbital more effec-tively than electrons in a 3s orbital. (d) the energy of the electron can be spread between three 3p orbitals instead of only one 3s orbital.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1381
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos