15. Chemical Kinetics
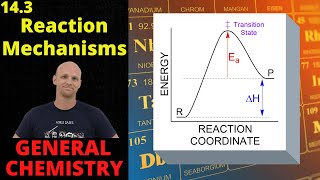
Reaction Mechanism
Problem 72a
Textbook Question
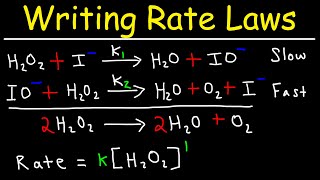
Textbook QuestionThe decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought to proceed by a two-step mechanism: H2O21aq2 + I - 1aq2¡H2O1l2 + IO- 1aq2 1slow2 IO- 1aq2 + H2O21aq2¡H2O1l2 + O21g2 + I - 1aq2 1fast2 (c) Assuming that the first step of the mechanism is rate determining, predict the rate law for the overall process.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1577
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos