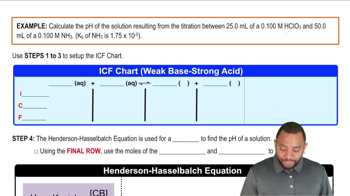
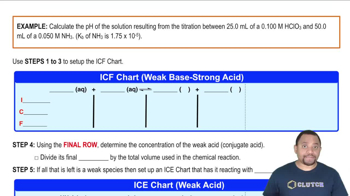
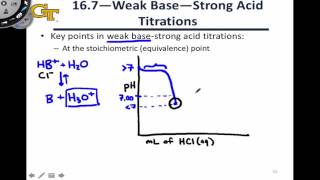
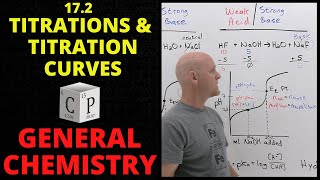
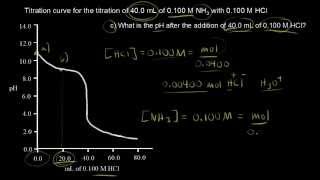
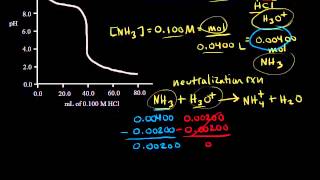
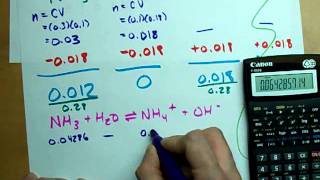18. Aqueous Equilibrium
Titrations: Weak Base-Strong Acid

Get help from an AI Tutor
Ask a question to get started.
Problem 55
Textbook Question
Textbook QuestionBlood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1). a. What is the pH of blood plasma?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1293
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos