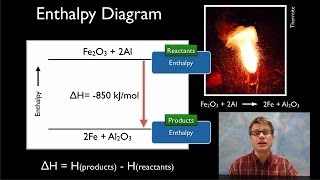
8. Thermochemistry
Enthalpy of Formation

Get help from an AI Tutor
Ask a question to get started.
Problem 152
Textbook Question
Textbook QuestionAcid spills are often neutralized with sodium carbonate or sodium hydrogen carbonate. For neutralization of acetic acid, the unbalanced equations are 112 CH3CO2H1l2 + Na2CO31s2 S CH3CO2Na1aq2 + CO21g2 + H2O1l2 122 CH3CO2H1l2 + NaHCO31s2 CH3CO2Na1aq2 + CO21g2 + H2O1l2 (c) How much heat in kilojoules is absorbed or liberated in each reaction? See Appendix B for standard heats of for- mation; ΔH°f = - 726.1 kJ>mol for CH3CO2 Na(aq).
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
19mPlay a video:
446
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 6 videos