18. Aqueous Equilibrium
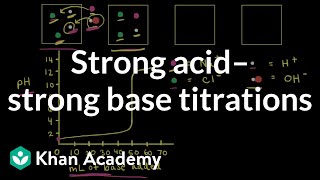
Titrations: Strong Acid-Strong Base

Get help from an AI Tutor
Ask a question to get started.
Problem 89
Textbook Question
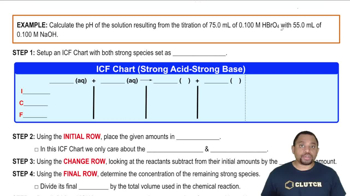
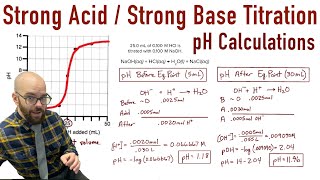
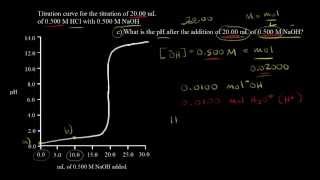
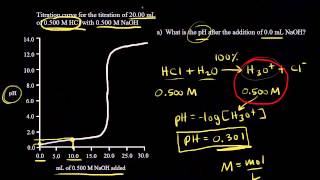
Textbook QuestionA 25.00-mL sample of an unknown HClO4 solution requires titration with 22.62 mL of 0.2000 M NaOH to reach the equivalence point. What is the concentration of the unknown HClO4 solution? The neutralization reaction is HClO4(aq) + NaOH(aq)¡H2O(l ) + NaClO4(aq)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
1980
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos