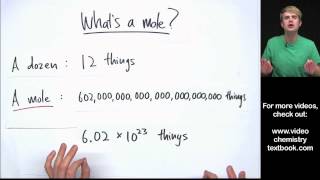2. Atoms & Elements
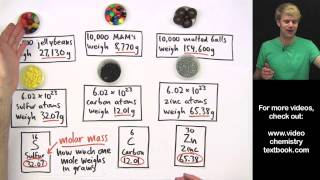
Mole Concept
Problem 57a
Textbook Question
Textbook QuestionWhat is the mass in grams of each of the following samples? (c) 0.0015 mol of diazepam (Valium), C16H13ClN2O
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
926
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 15 videos